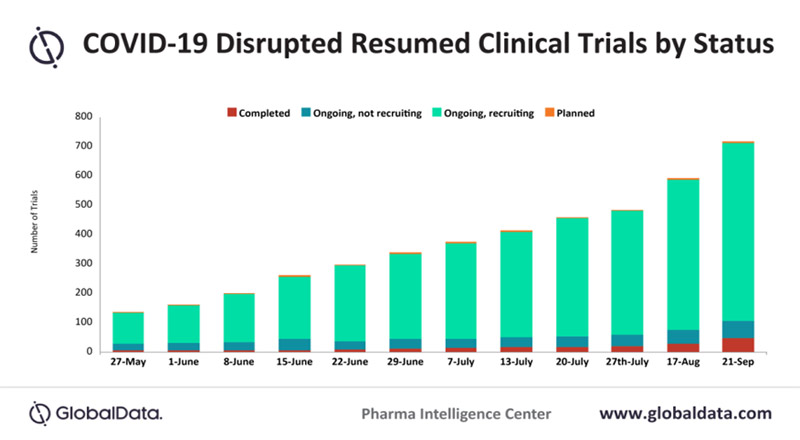
Clinical trials disrupted by COVID-19 are expected to resume at a much slower rate than originally anticipated, as more nations are re-enforcing lockdown due to a globally rising number of COVID-19 cases, says GlobalData, a leading data and analytics company.
Priya Nair, Trials Intelligence Analyst at GlobalData, comments: “The general trend shows a gradual increase in the overall percentage of trials for each trial status*, the biggest of which has been seen in trials that are ongoing and recruiting and those that are ongoing but not recruiting. Between 17 August and 21 September, ongoing and recruiting trials decreased from 84.7% to 82.2%, while completed trials increased from 4.5% to 6.4%.”
The number of resumed trials increased from over 600 on 17 August to 741 as of 21 September. Out of these trials, 82.2% are currently recruiting participants, 7.9% have completed recruitment but are still ongoing, and 0.5% of trials have yet to start recruiting subjects.
There is an overall steady increase of trials resuming activity. The US has the highest number of resumed trials at 70.6%, followed by France at 7.6%, the UK and Spain at 7.3% each, and Japan at 7.2%.
Nair concludes: “As large numbers of companies shift to alternative ways to conduct trials, it is possible that the use of virtual trials may still be prominent even after the COVID-19 pandemic ends.”
* Completed; Ongoing, not recruiting; Ongoing, recruiting; Planned